Sunday, 13 October 2013
Black hole of calcutta historical accident
The Black Hole of Calcutta was a small dungeon in the old Fort William in Calcutta, India, where troops of the Nawab of Bengal, Siraj ud-Daulah, held British prisoners of war after the capture of the fort on 20 June 1756.
One of the prisoners, John Zephaniah Holwell, claimed that following the fall of the fort, British and Anglo-Indian soldiers and civilians were held overnight in conditions so cramped that many died from suffocation, heat exhaustion and crushing. He claimed that 123 prisoners died out of 146 held. However, the precise number of deaths, and the accuracy of Holwell's claims, have been the subject of controversyFort William was established to protect the East India Company's trade in the city of Calcutta, the principal town of the Bengal Presidency. In 1756, with the possibility of conflict with French forces, the British began building up the fort's strengths and defences. The Nawab of Bengal, Siraj ud-Daulah, was unhappy with the company's interference in the internal affairs of his province and perceived a threat to its independence. He ordered an immediate stop to the Fort's military enhancement, but the Company paid no heed. As a consequence, Siraj organized his army and laid siege to the fort. The garrison's commander organised an escape, leaving behind 146 soldiers[2] under the command of John Zephaniah Holwell, a senior East India Company bureaucrat who had been a military surgeon. However, desertions by allied troops made even this temporary defence ineffectual, and the fort fell on 20 June. The surviving defenders, who numbered between 64 and 69, were captured along with an unknown number of Anglo-Indian soldiers and civilians who had been sheltering in the fort. During this period some prisoners were able to escape.Holwell wrote about the events after the fall of the fort. He met with Siraj, who assured him "on the word of a soldier [sic], that no harm should come to us".[3] After seeking a place in the fort to confine the 146 prisoners (including Holwell), at 8 pm, the jailers locked the prisoners in the fort's prison ("the black hole" was 18th century military slang for any military prison - similar to "the glasshouse" in the 20th century British Army or "the brig" in the US Navy),[4] which was 14 by 18 feet (4.3 m × 5.5 m) in size. When the "Black Hole" was opened the next morning at 6 am, only 23 people were alive.[2] Stanley Wolpert argues that only 64 people were imprisoned and 21 survived.[2] D.L. Prior argues that 43 members of the garrison were dead or missing for reasons other than suffocation and shock,[5] while Busteed argues that, because so many non-combatants were present in the fort when it fell, the number who died cannot be stated with any precision.[6] Regarding responsibility, Holwell believed that it "was the result of revenge and resentment in the breasts of the lower Jemmaatdaars [sergeants], to whose custody we were delivered, for the number of their order killed during the siege."[3] Wolpert concurs and argues that Siraj did not order it and was not informed about it.[2]The Holwell account
Description of Chakor Partridge"National Bird"
The Chakor partridge or Chakor Eurasian upland gamebird in the pheasant family Phasianidea. It has been considered to form a superspecies complex along with the Rock partridge, Phiby's partridge. Chakor is the national bird of Pakistan. His scientific name is Alectoris chakur. Its classification is Alectoris. Chakor partridge has well marked black and white bars on the flanks and a black band running from the forehead across the eye and running down the head to form a necklace that encloses a white throat. The chakor is the rotund 32-35 cm long partridge. It is very similar to Rock partridge (Alectoris graeca).
Description of Markhor "National Animal"

The markhor is the large species of wild goat that is found in northeastern Afghanistan , northern Pakistan, some parts of Indian Jammu kashmir, southern Tajikistan, southern Uzbekistan. Markhor is the national animal of Pakistan. His scientific name is capra felconeri and its classification is capra. Markhor are 65 to 115 cm tall at the shoulder. They usually weigh from 40 to 110 kg. Female are a tan color with a white underbelly and a pattern of black and white on legs. Male are the lighter tan color with a same white underbelly and pattern on the legs male also have a black face. Both males and females have corkscrew-shaped horns which can grow up to 160 cm / 64 inches long in males, and up to 25 cm / 10 inches long in female. The word "markhor" is persian for "snake eater".
Biography and qoute of Adolf Hitler



Barmuda Triangle

The Bermuda Triangle, also known as the Devil's Triangle, is an undefined region in the western part of the North Atlantic Ocean, where a number of aircraft and ships are said to have disappeared under mysterious circumstances.
Location:
Pakistan
Lewis Acid and Base and interaction matrix
Theory of Lewis Acid and Base:
| The Lewis Theory of acids and basesThis theory extends well beyond the things you normally think of as acids and bases. The theory
Lewis bases It is easiest to see the relationship by looking at exactly what Bronsted-Lowry bases do when they accept hydrogen ions. Three Bronsted-Lowry bases we've looked at are hydroxide ions, ammonia and water, and they are typical of all the rest. 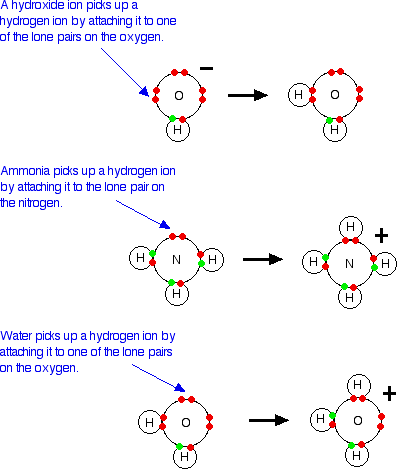 So how does this extend the concept of a base? At the moment it doesn't - it just looks at it from a different angle. But what about other similar reactions of ammonia or water, for example? On the Lewis theory, any reaction in which the ammonia or water used their lone pairs of electrons to form a co-ordinate bond would be counted as them acting as a base. Here is a reaction which you will find talked about on the page dealing with co-ordinate bonding. Ammonia reacts with BF3 by using its lone pair to form a co-ordinate bond with the empty orbital on the boron. 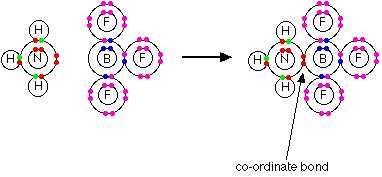 | |
Note: If you haven't already read the page about co-ordinate bonding you should do so now. You will find an important example of water acting as a Lewis base as well as this example - although the term Lewis base isn't used on that page.Use the BACK button on your browser to return quickly to this page. | |
| Lewis acids Lewis acids are electron pair acceptors. In the above example, the BF3 is acting as the Lewis acid by accepting the nitrogen's lone pair. On the Bronsted-Lowry theory, the BF3 has nothing remotely acidic about it. This is an extension of the term acid well beyond any common use. What about more obviously acid-base reactions - like, for example, the reaction between ammonia and hydrogen chloride gas? What exactly is accepting the lone pair of electrons on the nitrogen. Textbooks often write this as if the ammonia is donating its lone pair to a hydrogen ion - a simple proton with no electrons around it. That is misleading! You don't usually get free hydrogen ions in chemical systems. They are so reactive that they are always attached to something else. There aren't any uncombined hydrogen ions in HCl. There isn't an empty orbital anywhere on the HCl which can accept a pair of electrons. Why, then, is the HCl a Lewis acid? Chlorine is more electronegative than hydrogen, and that means that the hydrogen chloride will be a polar molecule. The electrons in the hydrogen-chlorine bond will be attracted towards the chlorine end, leaving the hydrogen slightly positive and the chlorine slightly negative. | |
Note: If you aren't sure about electronegativity and bond polarity it might be useful to follow this link.Use the BACK button on your browser to return quickly to this page. | |
| The lone pair on the nitrogen of an ammonia molecule is attracted to the slightly positive hydrogen atom in the HCl. As it approaches it, the electrons in the hydrogen-chlorine bond are repelled still further towards the chlorine. Eventually, a co-ordinate bond is formed between the nitrogen and the hydrogen, and the chlorine breaks away as a chloride ion. This is best shown using the "curly arrow" notation commonly used in organic reaction mechanisms. 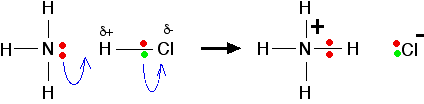 | |
Note: If you aren't happy about the use of curly arrows to show movements of electron pairs, you should follow this link.Use the BACK button on your browser to return quickly to this page. | |
| The whole HCl molecule is acting as a Lewis acid. It is accepting a pair of electrons from the ammonia, and in the process it breaks up. Lewis acids don't necessarily have to have an existing empty orbital | |
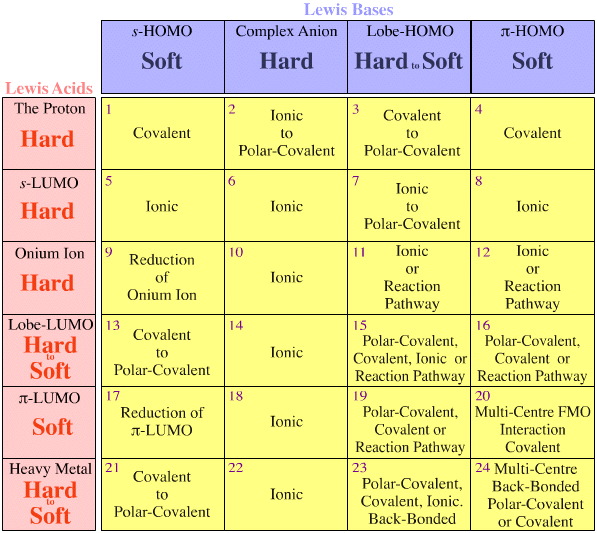
Lewis Acid Base Interaction Matrix
Sayings of Bill Gates.

Types of spherical mirror and their points.
Figure 1.1
Figure 1.2
Here we will study about the types of Spherical mirror. We know that Spherical mirror have two types:
- Concave mirror
- Convex mirror
Concave mirror:
Concave mirror is a spherical mirror whose inner curved surface reflecting. In concave mirror the size of image depends on the position of the object. Both virtual and real image can be formed by concave mirror.
Convex mirror:
Convex mirror is a spherical mirror whose outer curved surface is reflecting. In convex mirror the size of the image is always smaller than the object. Only virtual and erect image is formed by a convex mirror.
Another points of mirror are present below:
Pole:
It is the midpoint of the curved surface of spherical mirror. It is also called vertex.
Centre of Curvature:
A spherical mirror is a part of sphere. the centre of this sphere is known as Centre of Curvature.
Radius of Curvature:
It is the radius of the sphere from centre of curvature to spherical mirror.
Principal Axis:
It is the line which join centre of curvature to the pole of spherical mirror.
The Principal Focus:
The principal focus of concave and convex mirror is different. In concave mirrror, After reflection from a mirror, rays parallel to the principal axis converge to a Point F. This point if Principal Focus of concave mirror . Principal focus of concave mirror is also known as real focus. Int he case of convex mirror, rays parallel to the principal axis after reflection appear to come from a point F situated behind the mirror. This point is called the Principal focus of convex mirror. The principal Focus of the convex mirror is also known as virtual focus.
Focal Length:
It is the distance from the pole to the principal focus measured along the principal axis.
These all points you see in the above figure.
Subscribe to:
Comments (Atom)












