Theory of Lewis Acid and Base:
| The Lewis Theory of acids and basesThis theory extends well beyond the things you normally think of as acids and bases. The theory
Lewis bases It is easiest to see the relationship by looking at exactly what Bronsted-Lowry bases do when they accept hydrogen ions. Three Bronsted-Lowry bases we've looked at are hydroxide ions, ammonia and water, and they are typical of all the rest. 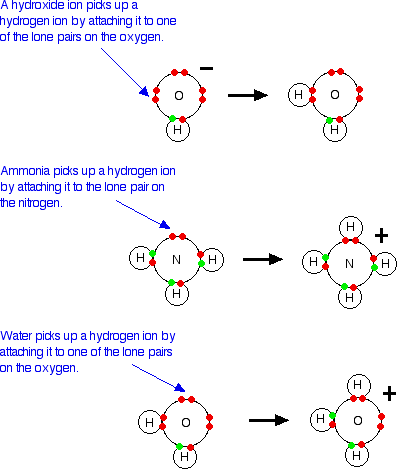 So how does this extend the concept of a base? At the moment it doesn't - it just looks at it from a different angle. But what about other similar reactions of ammonia or water, for example? On the Lewis theory, any reaction in which the ammonia or water used their lone pairs of electrons to form a co-ordinate bond would be counted as them acting as a base. Here is a reaction which you will find talked about on the page dealing with co-ordinate bonding. Ammonia reacts with BF3 by using its lone pair to form a co-ordinate bond with the empty orbital on the boron. 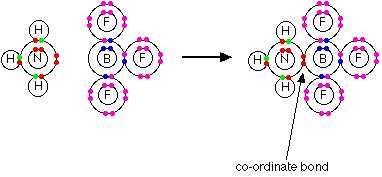 | |
Note: If you haven't already read the page about co-ordinate bonding you should do so now. You will find an important example of water acting as a Lewis base as well as this example - although the term Lewis base isn't used on that page.Use the BACK button on your browser to return quickly to this page. | |
| Lewis acids Lewis acids are electron pair acceptors. In the above example, the BF3 is acting as the Lewis acid by accepting the nitrogen's lone pair. On the Bronsted-Lowry theory, the BF3 has nothing remotely acidic about it. This is an extension of the term acid well beyond any common use. What about more obviously acid-base reactions - like, for example, the reaction between ammonia and hydrogen chloride gas? What exactly is accepting the lone pair of electrons on the nitrogen. Textbooks often write this as if the ammonia is donating its lone pair to a hydrogen ion - a simple proton with no electrons around it. That is misleading! You don't usually get free hydrogen ions in chemical systems. They are so reactive that they are always attached to something else. There aren't any uncombined hydrogen ions in HCl. There isn't an empty orbital anywhere on the HCl which can accept a pair of electrons. Why, then, is the HCl a Lewis acid? Chlorine is more electronegative than hydrogen, and that means that the hydrogen chloride will be a polar molecule. The electrons in the hydrogen-chlorine bond will be attracted towards the chlorine end, leaving the hydrogen slightly positive and the chlorine slightly negative. | |
Note: If you aren't sure about electronegativity and bond polarity it might be useful to follow this link.Use the BACK button on your browser to return quickly to this page. | |
| The lone pair on the nitrogen of an ammonia molecule is attracted to the slightly positive hydrogen atom in the HCl. As it approaches it, the electrons in the hydrogen-chlorine bond are repelled still further towards the chlorine. Eventually, a co-ordinate bond is formed between the nitrogen and the hydrogen, and the chlorine breaks away as a chloride ion. This is best shown using the "curly arrow" notation commonly used in organic reaction mechanisms. 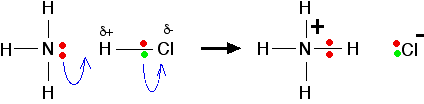 | |
Note: If you aren't happy about the use of curly arrows to show movements of electron pairs, you should follow this link.Use the BACK button on your browser to return quickly to this page. | |
| The whole HCl molecule is acting as a Lewis acid. It is accepting a pair of electrons from the ammonia, and in the process it breaks up. Lewis acids don't necessarily have to have an existing empty orbital | |
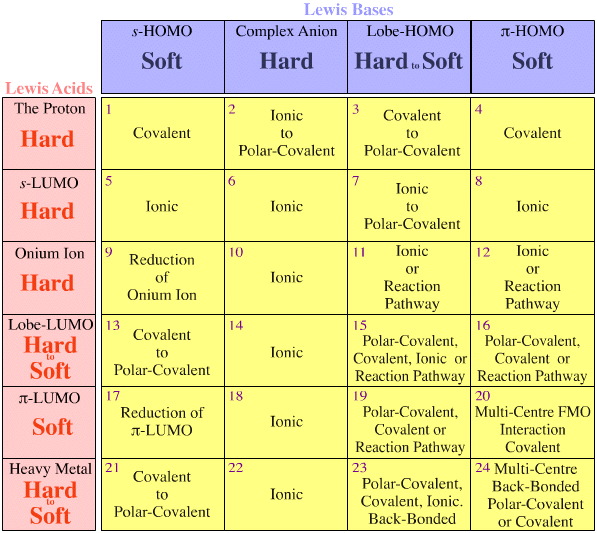
Lewis Acid Base Interaction Matrix
No comments:
Post a Comment